The preliminary indications for access to genomic sequencing correspond to specific clinical situations for which current knowledge indicates that whole genome sequencing provides greater benefit to patients than the techniques routinely used in genetics laboratories.
They serve as eligibility criteria for referring patients to national genomic sequencing laboratories (SeqOIA or AURAGEN) when this type of test is likely to provide significant diagnostic, prognostic or therapeutic benefit, and when conventional techniques prove insufficient.
 The list of pre-indications for which patients may benefit from genomic sequencing during their course of care is expanding over time, based on the transfer of scientific advances from research to care, following a validation phase by a working group led by the Haute Autorité de Santé (HAS). These pre-indications currently concern:
The list of pre-indications for which patients may benefit from genomic sequencing during their course of care is expanding over time, based on the transfer of scientific advances from research to care, following a validation phase by a working group led by the Haute Autorité de Santé (HAS). These pre-indications currently concern:
- Rare genetic diseases
- Hereditary predispositions to cancer
- Cancers
The long-term goal is to expand access to genomic sequencing for common diseases with a strong genetic component as knowledge advances.
See the list of pre-indications for access to genome sequencing
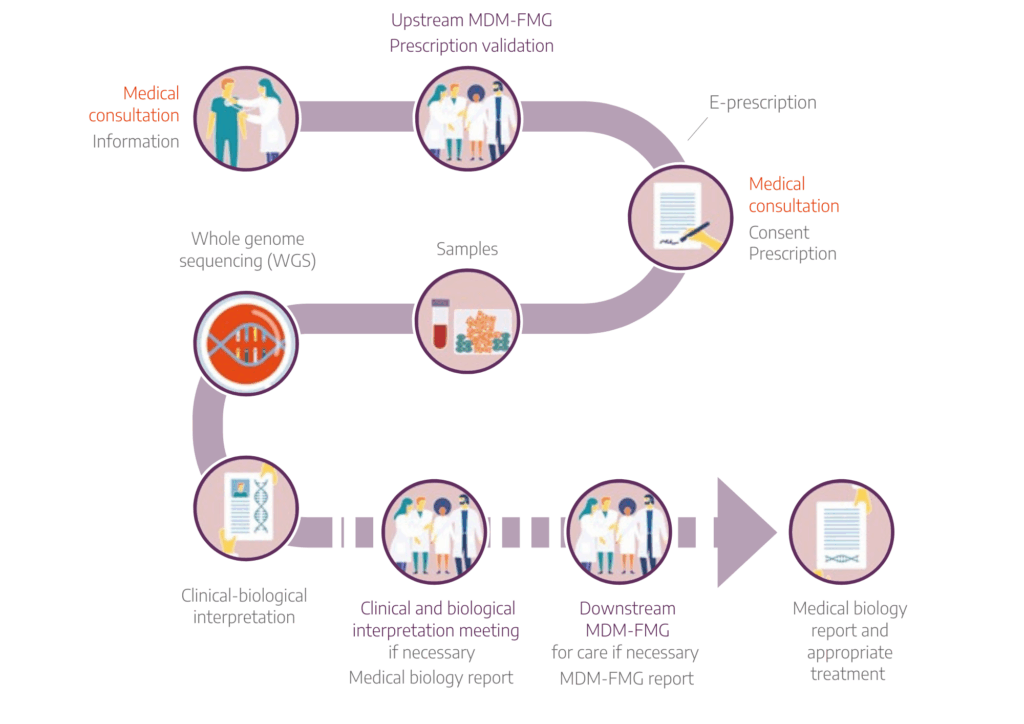
Genomic medicine care pathway :
MDM-FMG: PFMG Multidisciplinary Meetings to validate the medical indication and the conditions for carrying out the tests (e.g. relatives to be sampled, etc.) for the prescription of genomic sequencing tests carried out by the AURAGEN and SeqOIA laboratories
Role of pre-indications:
- Ensure equitable and justified access to genomic sequencing throughout the country.
- Prioritise clinical situations where data indicate that genomic sequencing provides a clinical benefit over standard techniques.
- Oversee the gradual roll-out of genomic sequencing in medical practices.
A working group led by the HAS is responsible for prioritising pre-indications for which patients can benefit from genomic sequencing during their care pathway (measure 6 of the PFMG2025). This working group also takes into account the technical constraints involved in sequencing and bioinformatic analysis of the results.
Five prioritisation campaigns have been organised since 2019. At the end of the fifth campaign in 2025, 82 pre-indications were validated:
- 67 for rare diseases
- 4 for hereditary predispositions to cancer
- 11 for cancers
The pre-indications selected will be evaluated by the HAS, which will issue an opinion on their reimbursement by the French national health insurance system. Data enabling the clinical efficacy and clinical and medico-economic utility of these innovative procedures to be validated are collected for each pre-indication. The work of PFMG2025 thus aims to validate the entry into a reimbursement process for the indication under study.
Where are we ?
Sorry, we couldn't find any news.

