Genomic medicine has profoundly changed personalised patient care. To ensure that everyone has equal access to new genomic medicine technologies throughout the country, France launched the 2025 France Genomic Medicine Initiative (PFMG2025) in 2016, following a request from the Prime Minister to the Aviesan Alliance. This plan aims to transform the way patients are diagnosed, prevented and treated by 2025.
The plan aims to integrate genomic medicine into clinical practice, with the ambition of providing equitable access to pan-genomic testing for all patients concerned.
Centred around the patient, PFMG2025 includes the implementation of a specific care pathway, the study and definition of new clinical indications to prioritise access to genomic medicine, and the establishment of dedicated infrastructure (LBM-FMG AURAGEN and SeqOIA, CreFiX and CAD). It also relies on cross-functional actions, such as the implementation of ‘pilot’ research projects, the deployment of a training plan, medico-economic evaluation, and the establishment of collaborations with international partners.
From an operational perspective, PFMG2025 is organised around three objectives :
Prepare for the integration of genomic medicine into routine care pathways and the management of diseases. The aim is to guarantee access to genomic medicine for patients who need it, whether they have cancer, a rare disease or, in the long term, a common disease.
Establish a national genomic medicine network for patients, capable of driving scientific and technological innovation, industrial development and economic growth.
To place France at the forefront of the major countries engaged in personalised medicine, with the capacity to export the expertise of our medical and industrial sector in genomic medicine.
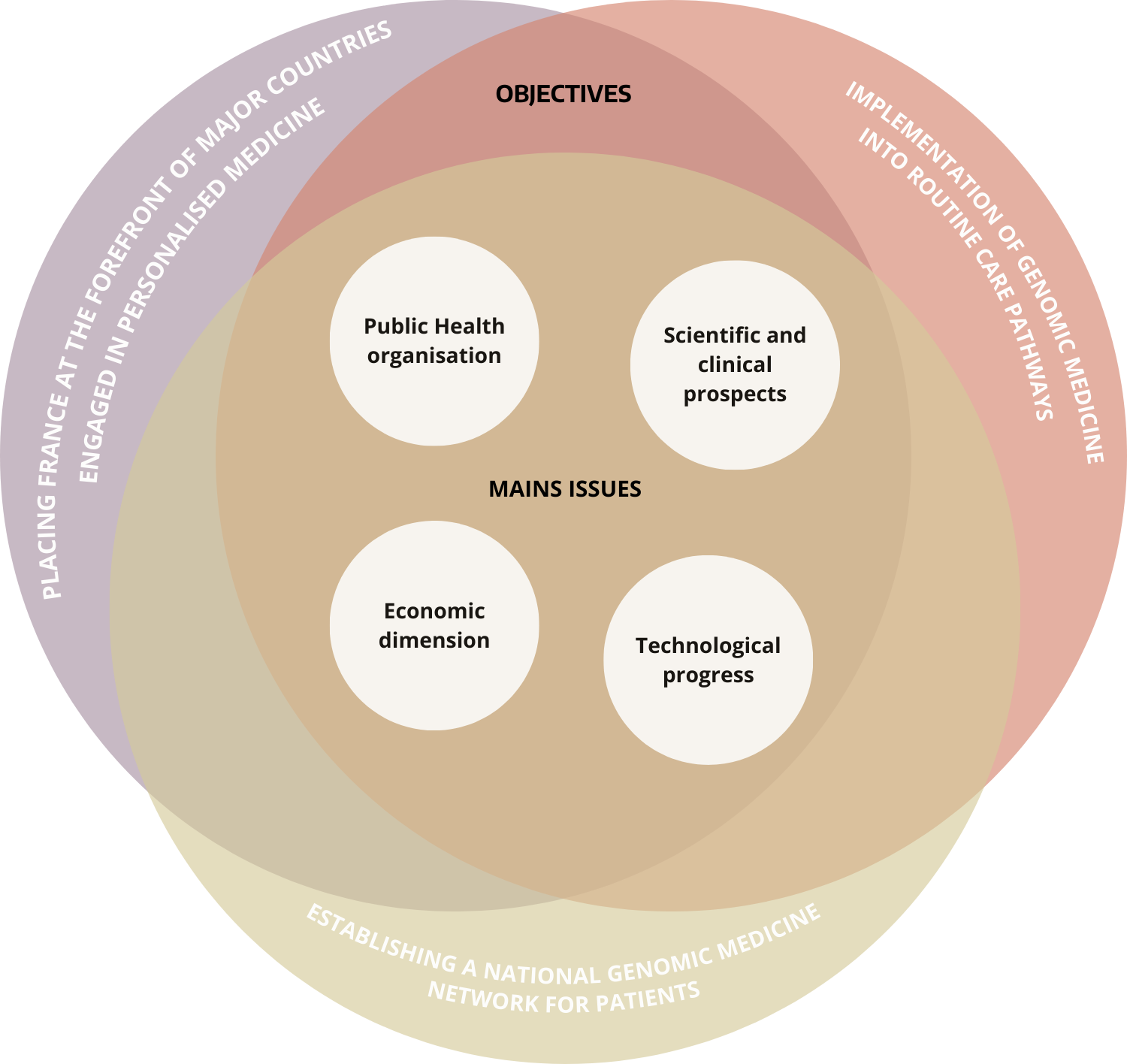
The plan addresses 4 major issues :
Genomic medicine is revolutionising patient care pathways. Routine genome sequencing enables patients to receive personalised diagnostic and therapeutic care. This already applies to patients affected by certain rare diseases or cancers; ultimately, the aim of PFMG2025 is to extend access to genomic sequencing and personalised care to patients with more common diseases.
The aim is to structure and strengthen the continuum between care and research by accelerating research through the reuse of genomic data to improve understanding of diseases and develop new treatments, then transferring this new knowledge to care in order to improve diagnosis and patient management.
New technologies are set to converge with life and health sciences. The ability to acquire, store, distribute, match and interpret massive genomic data is at the heart of this convergence.
Finally, there is an economic challenge, not only in terms of the sustainability of costs for our healthcare system, but also as a strategic opportunity to develop and structure a new industrial sector around partners already present in the field.
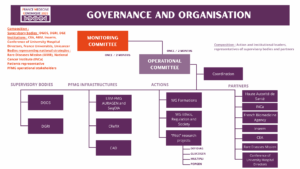
PFMG2025 is governed by a monitoring committee made up of representatives from the Plan’s supervisory bodies, partner institutions, organisations representing national strategies (rare diseases, cancer), a patient representative, and operational stakeholders in the PFMG2025. This committee meets every two months and is supported by an Operational Committee (COMOP), made up of action leaders and institutions, as well as representatives of the supervisory authorities and partners involved.